One of the most difficult sections of the new HSC Chemistry syllabus is spectroscopy in Module 8
This includes nuclear magnetic resonance (NMR), infrared (IR), ultraviolet (UV) spectroscopies and mass spectrometry.
In this entry, I’ll be diving deep into mass spectrometry to give you an idea of how much detail you’ll need to know, to be able to describe this technique, as well as analyse given results in the HSC exam.
What is mass spectrometry and what is it used for?
Mass spectrometry is a technique used to analyse and identify unknown compounds by measuring the mass to charge ratio![]() of fragment ions. It can be used to determine the chemical structure of an unknown sample of identifying functional groups and components within the compound.
of fragment ions. It can be used to determine the chemical structure of an unknown sample of identifying functional groups and components within the compound.
What does the machine look like and how does it work?
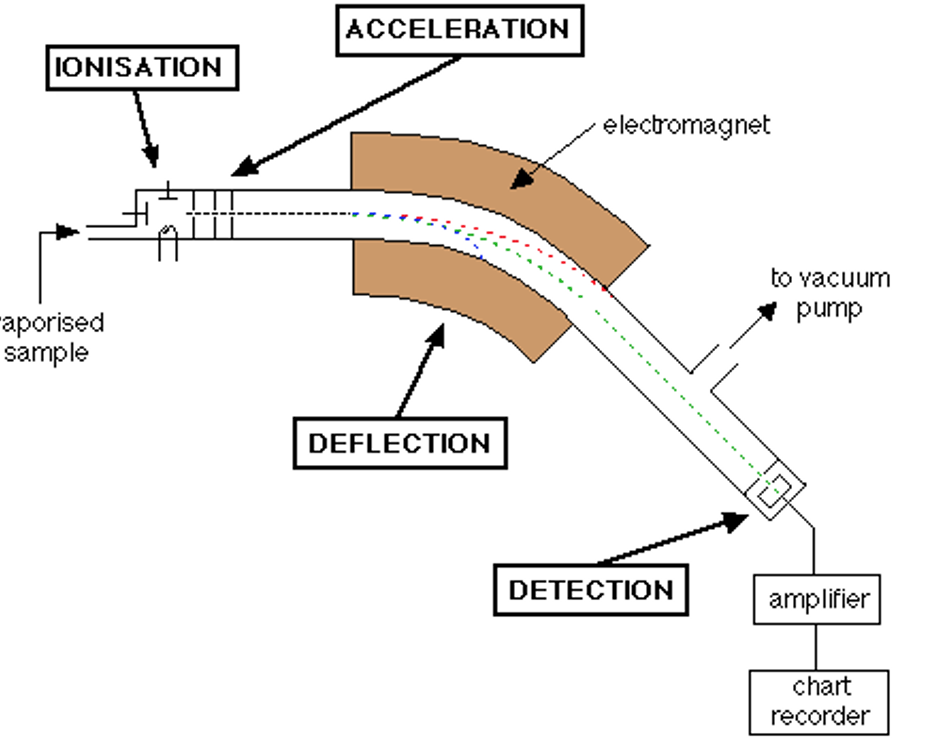
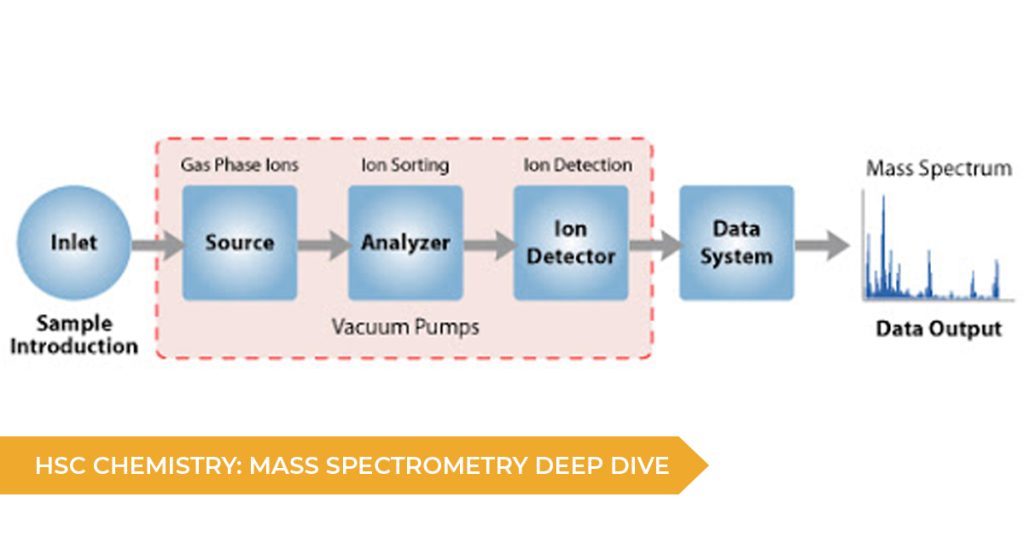
A mass spectrometer contains three key components:
– A method of ionising the sample. This removes electrons from the molecules and also breaks them down into smaller fragments. In the end, each fragment has a unique mass to charge ratio that can be detected. These fragments are accelerated to create a beam of ions.
– A magnetic array to deflect and separate ions based on the mass to charge ratio. Lighter ions are deflected more than heavier ions. The magnetic field strength can be adjusted to focus different masses at the detector.
RELATED: Breaking Down The HSC Chemistry Formula Sheet
– A detector that measures the mass to charge ratio of the focused ions. By testing a variety of different magnetic field strengths, a full graph can be developed.
Additionally, this process must be conducted under a vacuum to prevent ions reacting with particles in the air and interfering with the measurements.
How do I interpret a mass spectrometer graph?
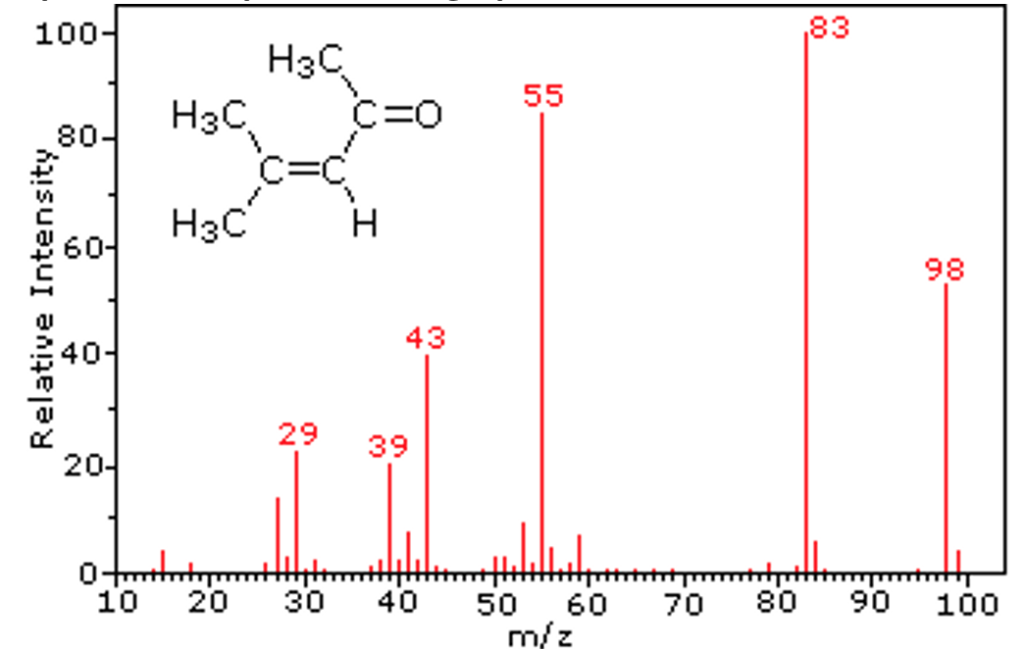
This graph shows the output of a mass spectrometer for the compound shown in the top left. There are several feature of a mass spectrometer graph that you need to be familiar with:
– The molecular ion: This is the ion with the highest mass to charge ratio, in this case 98. This represents the molar mass of the compound ![]()
– The base peak: This is the most intense ion in the graph, in this case 83. This tells us that it is more likely that the molecular fragmented when ionised, than staying as a full molecular ion. In this case, it represents the loss of an ion of 15 mass units, which corresponds to a methyl group ![]() This group can also be seen by the small peak at 15.
This group can also be seen by the small peak at 15.
This is an important concept – each peak also corresponds to another peak representing the rest of the molecule after a fragment is removed.
– Other common ion peaks: The other labelled peaks in the graph must represent another fragment of the original molecule. The table below summarises the likely fragments associated with each peak:
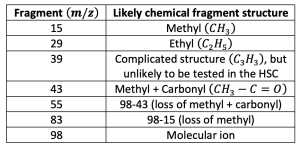
With this information, we can piece together the overall molecule. However, it may be difficult to fully confirm the molecular structure without another technique. This is why mass spectrometry is often used in conjunction with IR or NMR spectroscopy.
Need some extra help? We’ve got you covered at Talent 100
We have learning centres in Sydney (Burwood, Chatswood, Epping, Hurstville & Sydney CBD), so you can brush-up on your Chemistry skills before exams start. We’ve also created our own handy Pocket Guide containing data sheets for Mathematics, Physics and Chemistry – they can be found by asking our Student Services team at the front desk!
We also have online classes available for students in NSW – so you won’t miss out on any valuable Chemistry time this year! Take advantage of our 1-1 classes where you can get your past papers marked, ask questions about homework, or just speak to one of our HSC Chemistry Mentors.
Click here to find out more about our HSC Chemistry tuition courses.
Written by our Talent 100 HSC Chemistry Mentor, Chris Skellern.




