Breaking down the HSC Chemistry Data Sheet
Apart from the exam paper, in the HSC you’ll also be given a 4-page document with various essential formulas and data values that you may need to refer to.
It is important that you understand where and when you might need to use these values to avoid having to memorise unnecessary amounts of information. In this entry, I’d like to run through each section of this data sheet and point out where they become relevant in each of the modules of the HSC Chemistry course.
Page 1: Formulae Sheet and Solubility Constants
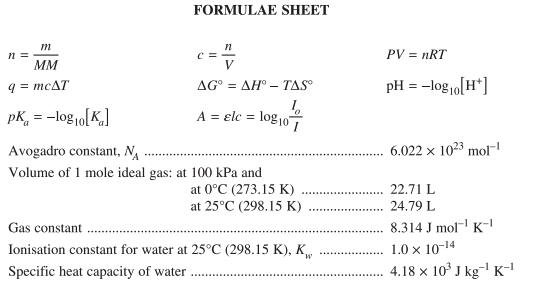
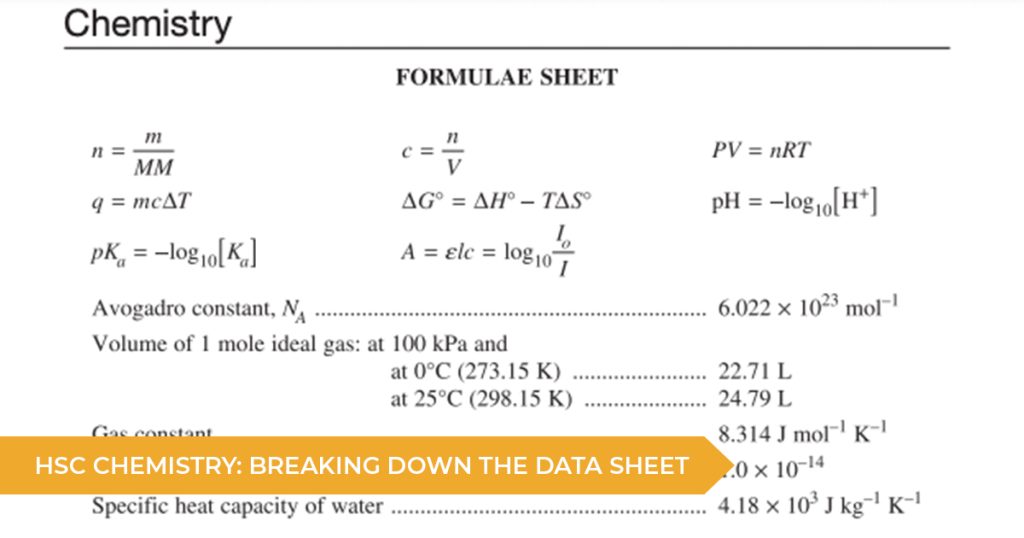
The first page contains a list of formulae and constants from across the course. Formulas like those for pKa and pH come directly from Module 6, while formulas for Gibbs free energy come directly from Module 5.
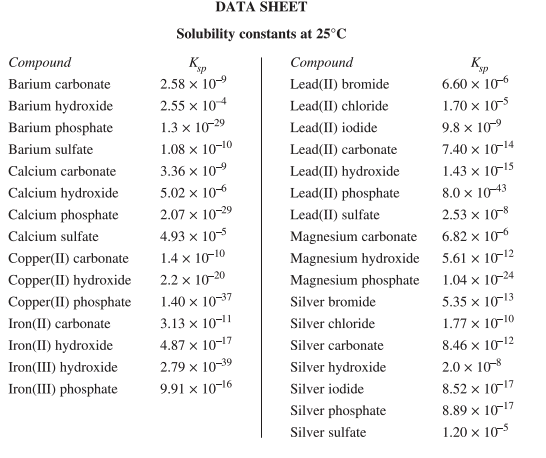
Next, a list of solubility constants that come from the last section of Module 8. To apply these constants, you’ll need to know the formula for the solubility constant. For example, for silver carbonate:
![]()
Page 2: Infrared absorption, 13Carbon NMR chemical shift and UV absorption data
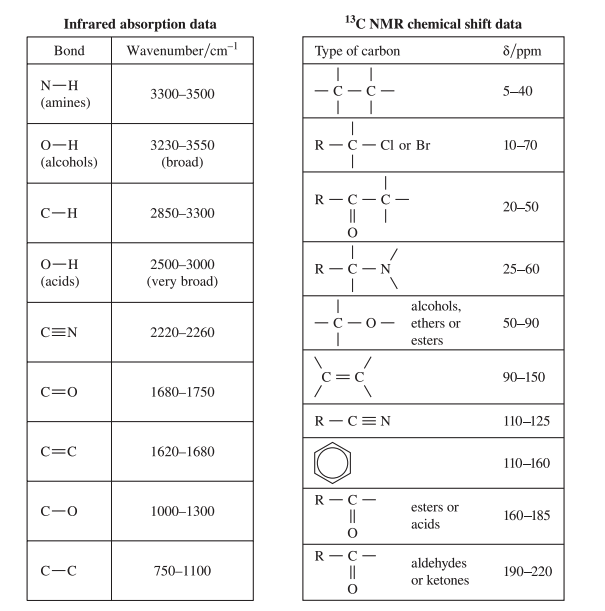
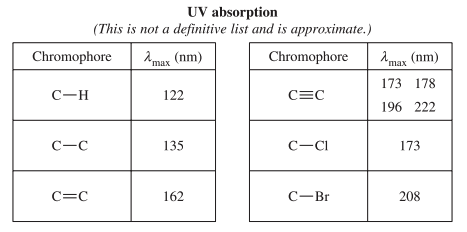
The second page is purely from Module 8 and contains a list of functional groups and corresponding numbers. These numbers describe where each of the functional groups would appear in graphs from these three spectroscopic techniques ![]()
RELATED: The Ultimate Guide To HSC Chemistry – Modules 5 – 8
You’ll need to be aware of what each of the numbers represents:
![]()
Wavenumber is the inverse of wavelength and represents the energy of infrared radiation absorbed by a sample. Infrared radiation is absorbed by bonds in functional groups like amines and alcohols by changing how the bond vibrates or rotates.
![]()
Chemical shift describes how a nucleus respond to a magnetic field relative to some standard. For the HSC, that standard (at 0 ppm) is TMS (tetramethylsilane). Any nucleus close to an electronegative atom like oxygen or nitrogen will be shifted from this standard. For example, carbon atoms in the carbonyl group of an aldehyde or ketone are shifted strongly to 190-220 ppm.
![]()
This lambda represents the peak of maximum UV radiation absorbance by a bond, similar to IR absorption. The data sheet only provides a few values for UV absorption, typically in highly polar bonds like C-CI or multiple bonds like C=C.
Page 3: Standard potentials

The third page is more commonly used in the Year 11 course where we study galvanic cells and metal reactivity, so rarely is required for the HSC exam.
Page 4: Periodic Table
Finally, the fourth page gives us the periodic table of the elements. Most of this you will likely know off by heart by the time you sit your HSC exam, but it is still a valuable resource for several important pieces of information including:
– Molar masses for mole calculations, especially in Module 6 and for fragments in mass spectroscopy from Module 8.
– Valency or ionic charges, especially for confirming correct ionic formulas in Module 5 and bonding patterns or oxidation numbers in Module 7.
Need some extra help? We’ve got you covered at Talent 100
We have learning centres in Sydney (Burwood, Chatswood, Epping, Hurstville & Sydney CBD), so you can brush-up on your Chemistry skills before exams start. We’ve also created our own handy Pocket Guide containing data sheets for Mathematics, Physics and Chemistry – they can be found by asking our Student Services team at the front desk!
We also have online classes available for students in NSW – so you won’t miss out on any valuable Chemistry time this year! Take advantage of our 1-1 classes where you can get your past papers marked, ask questions about homework, or just speak to one of our HSC Chemistry Mentors.
Click here to find out more about our HSC Chemistry tuition courses.
Written by our Talent 100 HSC Chemistry Mentor, Chris Skellern.