How to start naming chemicals in Organic Chemistry (Module 7)
The third module of the HSC Chemistry course, Module 7: Organic Chemistry, is the longest of the four modules, with 2.5 pages full of syllabus dot points.
It may seem daunting at first, and initially it seems like it will require significant memorisation, however the entire module is built on a set of simple rules that can be applied to all situations. In this entry, I’d like to outline the patterns and rules in the first syllabus dot point – “Investigate the nomenclature of organic chemicals” so you can see that it can be fully learnt without requiring any memorisation.
What chemicals can be assessed?
The syllabus identifies eight series of chemicals that you can be assessed on:
– Alkanes (carbon-carbon single bonds only)
– Alkenes (carbon-carbon double bonds)
– Alkynes (carbon-carbon triple bonds)
– Alcohols (hydroxy functional groups, O-H)
– Aldehydes and ketones (carbonyl functional groups, C=O)
– Carboxylic acids (carboxyl functional groups, COOH)
– Amines and amides (nitrogen groups, NH2, CONH2)
– Halogenated organic compounds (F, CI, Br, I)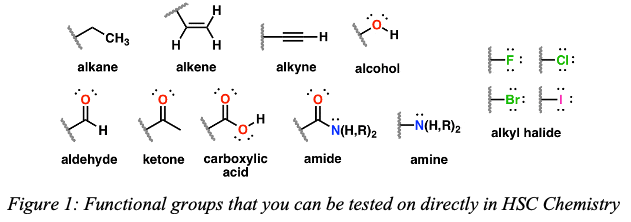
However, there are also a few other hints included in the wording of the syllabus dot point. These are:
– “Up to C8” So you can only be tested on carbon chains from 1 to 8 carbons long. The table below is a reminder of the prefixes associated with these chain lengths: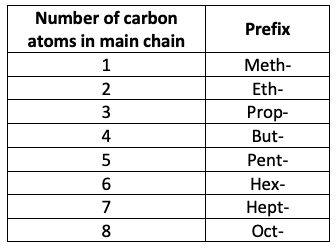
An easy way to remember these is to memorise the first four prefixes, then notice that from 5 onwards, it follows geometry (e.g. pentagon, octagon, etc)
– “Including simple methyl and ethyl branched chains” This tells us that we could also be tested on molecules that have a primary chain from 1 to 8 carbons long, but that there may be additional carbons in methyl or ethyl groups branching off this main chain.
RELATED: Band 6 HSC Chemistry Exam Tips: How To Ace Your Chemistry Exam
Which chemicals cannot be assessed?
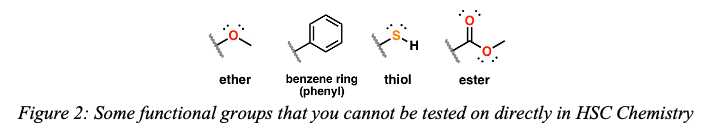
After all this, you’ll notice that sometimes you may learn about examples of compounds that do not fall into these series. These cannot be tested directly in the HSC but can still be useful to know about just for your general interest, or for reference in some of your practicals. The groups that you cannot be tested on include:
– Ethers (C-O-C bonds)
– Cycloalkanes (e.g cyclohexane), However, you may refer to use the cyclohexane and cyclohexene in Module 8 when conducting a practical to distinguish alkanes and alkenes.
– Benzene (C6H6 cycle with alternating single and double bonds)
– Thiols (carbon-sulfur bonds)
– Esters (COOC bonds). However, esterification is still a reaction you will become familiar with in Module 6, and it will be useful to know a few examples for this (e.g. methyl ethanoate).
You also cannot be tested on molecules with two or more of these functional groups in the same molecule, or with multiple of each functional group. For example:
– Diols (two alcohol groups in the same molecule)
– Enols (molecules with both a double bond and a hydroxy group in the same molecule)
How should I start learning how to name chemicals?
The simplest way to think about nomenclature for chemicals is to break it down into three main steps:
– Figure out the length of the main carbon chain to get your prefix. E.g. if you have 4 carbons in the main chain, your required prefix is “but-” (see table above)
– Figure out what the most important functional group is to get your suffix. E.g. if the main functional group was a carboxylic acid, your required suffix is “-anoic acid.” Hence, your final molecule in the previous example would be “butanoic acid” by combining the prefix and suffix.
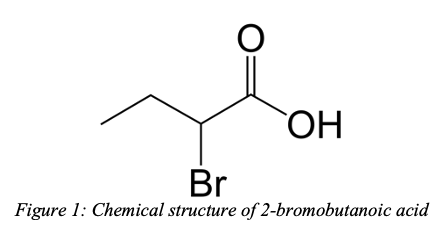
– Add any additional halogens or branched chains as an additional prefix with an associated number. E.g. if you had a bromine on the 2nd carbon in the previous example, your final molecule would be 2-bromobutanoic acid. This would be the below molecule:
Need some extra help? We’ve got you covered at Talent 100
All of our Sydney learning centres are now open in Term 3 (Burwood, Chatswood, Epping, Hurstville & Sydney CBD), so you can brush-up on your Chemistry skills before exams start. We’ve also created our own handy Pocket Guide containing data sheets for Mathematics, Physics and Chemistry – they can be found by asking our Student Services team at the front desk!
We also have online classes available for students in NSW – so you won’t miss out on any valuable Chemistry time this year! Take advantage of our 1-1 classes where you can get your past papers marked, ask questions about homework, or just speak to one of our HSC Chemistry Mentors.
Click here to find out more about our HSC Chemistry tuition courses.
Written by our Talent 100 HSC Chemistry Mentor, Chris Skellern.