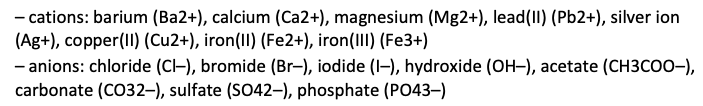Chemistry is a practical subject, and as such much of it is conducted in a lab
Although the final exam is still purely written, the practical aspect of the course can still be examined in several ways.
Additionally, one of the internal assessments and the depth study often involve a significant amount of practical work.
In this entry, I’d like to review the areas of the HSC Chemistry syllabus which require practical work, and identify the potential ways in which they can be examined in the written final HSC exam.
Which syllabus areas incorporate practical work?
Every module has some syllabus dot points which relate directly to practical work. They typically use phrases like “conduct practical investigations” or “conduct an investigation” to indicate that you could research these topics with a practical component, as opposed to purely theoretical work.
The relevant dot points for each module are shown below. Some interesting points to not are that Module 6 has the most practical work involved, while Module 8 is well over 50% based on practical work.
You may not get to do all of these in class, but you will still be expected to know how to respond to questions about them.
Module 5:
– Conduct practical investigations to analyse the reversibility of chemical reactions, for example: cobalt(II) chloride hydrated and dehydrated, iron(III) nitrate and potassium thiocyanate, burning magnesium and burning steel wool.
– Conduct an investigation to determine Keq of a chemical equilibrium system, for example: Keq of the iron(III) thiocyanate equilibrium.
– Conduct an investigation to determine solubility rules, and predict and analyse the composition of substances when two ionic solutions are mixed, for example: potassium chloride and silver nitrate, potassium iodide and lead nitrate, sodium sulfate and barium nitrate.
Module 6:
– Conduct an investigation to demonstrate the preparation and use of indicators as illustrators of the characteristics and properties of acids and bases and their reversible reactions.
– Conduct a practical investigation to measure the enthalpy of neutralisation.
– Conduct a practical investigation to measure the pH of a range of acids and bases.
RELATED: Mass Spectrometry Deep Dive
– Conduct a practical investigation to demonstrate the use of pH to indicate the differences between the strength of acids and bases.
– Conduct a chemical analysis of a common household substance for its acidity or basicity, for example: soft drink, wine, juice or medicine.
– Conduct a practical investigation to prepare a buffer and demonstrate its properties.
Module 7:
– Conduct a practical investigation to compare the properties of organic chemical compounds within a homologous series, and explain these differences in terms of bonding.
– Conduct a practical investigation to measure and reliably compare the enthalpy of combustion for a range of alcohols.
Module 8:
– Conduct qualitative investigations – using flame tests, precipitation and complexation reactions as appropriate – to test for the presence in aqueous solution of the following ions:
– Conduct investigations and/or process data involving: gravimetric analysis, precipitation titrations.
– Conduct investigations and/or process data to determine the concentration of coloured species and/or metal ions in aqueous solutions, including but not limited to the use of: colourimetry, ultraviolet-visible spectrophotometry, atomic absorption spectroscopy.
– Conduct qualitative investigations to test for the presence in organic molecules of the following functional groups: carbon–carbon double bonds, hydroxyl groups and carboxylic acids.
How can these areas be examined?
There are three primary ways in which practical investigations can be assessed in a written exam.
RELATED: How to use the HSC Chemistry Data Sheet
Firstly, questions can always be asked on the theory behind the investigations. For example, explaining what a buffer is from a purely theoretical perspective.
Secondly, it is important to understand and be able to describe the risks and mitigation methods in various investigations. For example, conducting flame tests with lead ions, or using concentrated acids and bases in Module 6 require detailed risk assessments that you should be prepared to describe.
Next, you can be expected to outline experimental methods used in conducting these investigations. These can be simply written in stepwise lists with the appropriate equipment and steps included. Finally, calculations and graphing based off provided results are very common questions and could be examined on all dot points, except those that specify “qualitative investigations.”
Need some extra help? We’ve got you covered at Talent 100
We have learning centres in Sydney (Burwood, Chatswood, Epping, Hurstville & Sydney CBD), so you can brush-up on your Chemistry skills before exams start. We’ve also created our own handy Pocket Guide containing data sheets for Mathematics, Physics and Chemistry – they can be found by asking our Student Services team at the front desk!
We also have online classes available for students in NSW – so you won’t miss out on any valuable Chemistry time this year! Take advantage of our 1-1 classes where you can get your past papers marked, ask questions about homework, or just speak to one of our HSC Chemistry Mentors.
Click here to find out more about our HSC Chemistry tuition courses.
Written by our Talent 100 HSC Chemistry Mentor, Chris Skellern.